Determining Substrate Orientation in the Active Site of a C-Nucleoside Synthase
Nigel Richards
Abstract
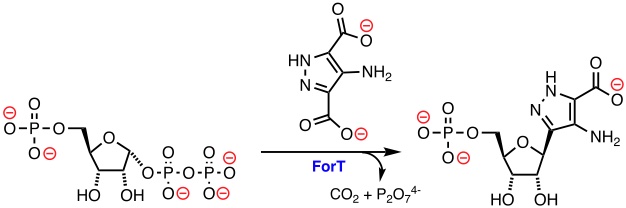
The biosynthetic enzyme, ForT, catalyzes the formation of a C-C bond between 4-amino-1H-pyrazoledicarboxylic acid and MgPRPP to produce a C-nucleoside precursor of formycin A. The transformation catalyzed by ForT is of chemical interest because it is one of only a few examples in which C-C bond formation takes place via an electrophilic substitution of a small, aromatic heterocycle. X-ray crystallography of the Michaelis and enzyme/product complexes provides conflicting information on the likely orientation of the heteroaromatic substrate within the active site of the enzyme. In this short talk I will discuss how MOPAC calculations were used to determine the most likely orientation of the heterocycle prior to C-C bond formation. These findings set the scene for structure-based strategies for the biocatalytic production of novel, anti-viral C-nucleoside and C-nucleotide analogs.